Executive War College 2024 Recap

We have returned from the 29th Annual Executive War College with renewed friendships, new connections, and some great memories. Our highlight was of course the excellent, informative panel headed by our own Robert Negosian, which could not have gone better thanks in no small part our two extremely knowledgeable panelists, Drs. Greg Sorensen and Andy Moye.
The gathering in New Orleans was as informative as it was captivating. There were many changes, both ‘on the ground’ in terms of new people attending, and ‘in the air’ in terms of the herculean efforts to harness the power of AI. And as always, everyone is watching what our government agencies are doing and speculating what their actions mean to our industry, including the benefits and the pitfalls. We’ll start there.
FDA Overreach?
The latest regulations, guidelines, and rules are always a hot topic at the EWC and this year was no exception. This was encapsulated in the panel “Timing and Implications of FDA LDT Rule; Reforms to CLIA Regulations; Wider Use of Z-Code by Payers,” chaired by William Morice, President and CEO of the Mayo Clinic. The FDA’s final ruling on Laboratory Developed Tests (LDTs), ironically, was released when I was traveling to the 2024 Executive War College — perfect timing for review, discussion, and concerns to be raised. The repercussions of this ruling remain to be seen with immediate concerns relating to how this will impact patient care, testing innovation and increased overall costs.
While the session was informative, it left some aspects unresolved. We do know that the impact on laboratory developed tests regarding oversight by the FDA is significant, to say the least. Understanding the challenges labs will face with the ever-changing rules and regulations is mission critical. We at U.S. HealthTek will be actively identifying how to best assist the laboratory community to be able to get through it all, so that we can continue to be a resource for the best solutions.
With these topics and more, it became clear that if there was one reason to attend this conference every year, it was to understand what changes in the law are affecting labs and how the industry is adjusting. Robert noted that while these conferences have traditionally been heavy in addressing compliance and billing issues, they haven’t traditionally been particularly “tech-heavy.” But with this conference, that did seem to change, and we are actively leading the charge on that.
Understanding AI
Our panel discussion on “The Real-World Impact of AI, Machine Learning, and Automation in Healthcare” could not have gone better. There were many sessions on AI, but Robert and Drs. Moye and Sorensen clearly laid out latest information on the topic, including the importance of building a viable data foundation, having a rock-solid plan from the start, and establishing a competent data model so your AI tools will deliver secure and accurate data.
An excerpt from our panel discussion on AI in Healthcare
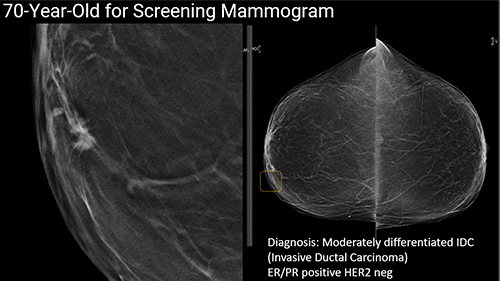
The panel discussed some excellent real-world success stories about how AI is making a difference in patient care. Dr. Sorensen had slides illustrating how AI today is to capture a higher percentage of diagnoses that might otherwise be missed for cancerous tumors. This was an especially exciting moment, as it opened discussions about the importance of having AI in the future to help streamline and automate processes. In light of the increasing shortage of certified pathologists, AI in this area is going to be especially critical.
Considering that people are living longer, with the biggest portion of the population bell curve starting to inch closer towards geriatric status, the healthcare community is going to need all the tools it can get, and the need for AI is going to increase. Yet, while we illustrated in our panel discussion that the solution is already here, it still needs to be ratified by payers, who as of now aren’t paying for AI testing. If it’s not covered by insurance, then patients who are willing to pay the out-of-pocket may benefit… but that brings up an equity issue.
And as with the most successful panel discussions, the questions and observations from the professionals in the audience were the best part. The session was interesting, informative, and engaging because it was truly a two-way discussion and everyone in the room shared ideas and thoughts about the potential and future of this technology.
All Work and No Play…
If there’s a distraction you need to take your mind off impending regulation and the challenges of AI, you’ll find it in NOLA. Acme Oyster House is always fun – what’s better than sneaking out of the conference and eating some fried food and having a cold beer? And a trip to NOLA is not complete without a visit to The Dungeon. This is a “speakeasy” that entices you with the chance of running into a vampire; alas, we were not so lucky. Maybe it’s the super-hard blaring rock that keeps them at bay? Yes, that aspect isn’t normally my cup of tea, but at midnight or 1am, it’s the place to stop on your way back to the hotel.

Karen Saldaña doing her very best Elvis
Want to know the best place to people watch in the entire town? I discovered it on the balcony on Bourbon Street at the Cat’s Meow! We co-hosted a karaoke night here with partners HealthReconnect and Wakefield, and it was an absolute success. Now, they told me that what happens in NOLA, stays in NOLA, but I took no chances and didn’t get up on myself. But I enjoyed everyone else’s performance and the talent on display was really impressive, starting with our own Karen Saldaña doing a notable Elvis impersonation singing “You Ain’t Nothin’ But a Hound Dog.” But Joretha Carodine from the Joint Pathology Center came in first place as she rocked Whitney Houston’s “Wanna Dance with Somebody.” We had a full house, and it was absolutely one of the best, most fun events we’ve ever had at EWC in our many years as a sponsor.
We’re Energized and We’re Here to Help
Now we’re back at work digesting what we learned, following up on conversations, and getting back to business. If there is one extremely urgent aspect of our industry that EWC illustrated for us, it is that it is ever-changing and requires diligence to remain on top of the latest technology and regulations. We will continue to keep up with those changes to the benefit of all our clients, and continue to lead the way in supporting our clients in healthcare and the laboratory industry.

